Featured Item
Experts take a stab at vaccine queries
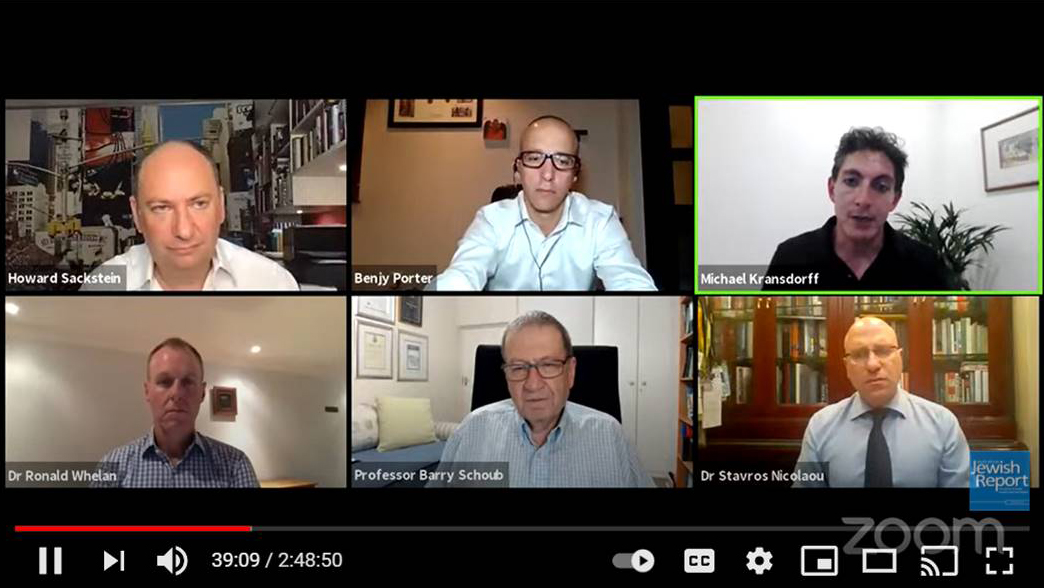
With the rollout of vaccines imminent in South Africa, a panel of local experts weighed in on the subject in a webinar hosted by the SA Jewish Report on Thursday, 21 January.
Aspen Pharmacare Group’s senior executive, Dr Stavros Nicolaou; the chairperson of the Ministerial Advisory Committee on the COVID-19 vaccine, Professor Barry Schoub; and the chief commercial officer of Discovery Health, Dr Ronald Whelan, answered questions posed by hundreds of viewers from around the world.
Q. What does vaccine efficacy mean?
A. Efficacy refers to the results in controlled trials. Volunteers are separated into groups of those who get the vaccine, and those who are given a placebo, and both groups are monitored to see whether the people given the vaccine are infected at a lower rate than those who get the placebo. If a vaccine is 90% effective, it means that if 100 people who were not previously infected by the coronavirus are given the vaccine, on average 90 of them won’t contract the virus.
Q. Will the vaccines be effective against the South African variant of the virus?
A. In spite of some concerns, at the moment it seems that the vaccine will most likely be effective. Some trial samples of post-vaccine blood haven’t neutralised the variant, while the Pfizer vaccine has been shown to effectively neutralise artificially constructed virus variants in studies conducted in the United States. According to the panel, this is a work in progress that may need to be studied closer at a later point but it won’t affect the rollout of vaccines in South Africa at the moment.
Q. What’s Aspen’s potential role in making vaccines in South Africa?
A. On the whole, there’s little vaccine manufacturing capability and capacity on the continent. Capacity for vaccine production is a rare and limited commodity in Africa, with rudimentary capability in Senegal and two facilities in South Africa, namely Aspen and BioVac. As part of its agreement with Johnson & Johnson (J&J), Aspen has repurposed some of its capacity for vaccine production (with capacity for 300 million doses a year). However, while it can formulate, fill, package, and label vials of the vaccine, it can’t create the active ingredient essential for the vaccine.
Q. Can the vaccine be purchased from Aspen?
A. After the trial process, only J&J can supply the vaccine. The decision about where the vaccine will be allocated remains that of J&J, which has estimated that the vaccine should become available to the global market in the second quarter of 2021.
Q. Will the J&J vaccine be a single or double-dose vaccine (the latter being the case with the Pfizer, Moderna, and AstraZeneca vaccines)? Does it matter?
A. J&J hasn’t released its data yet although we expect it to do so by the end of January. There are indications from J&J that it will be a single-dose vaccine. This is important because it reduces challenges on the African continent given the dispersion of the population in more remote areas. The single dose has other benefits such as compliance, reducing the cost of transportation and storage. If 40 million South Africans – 70% of the population – are to be vaccinated over 12 months, 170 000 vaccines would need to be administered per day (in a five-day working week), making a single dosage optimal.
Q. President Cyril Ramaphosa recently announced that South Africa has secured 30 million doses of the vaccine. What are they, and where are they coming from?
A. As reported, 1.5 million vaccine doses are expected over the next few weeks from the Serum Institute of India. There is a tranche coming from Covax (the international pool-procurement mechanism) which will provide about 12 million doses, along with a further 12 million doses secured from the African Union via Covax. J&J, too, has reportedly committed to supplying South Africa with nine million doses, bringing the total to about 34 million doses (a bouquet of AstraZeneca, Pfizer, and J&J vaccines). According to the panel, this is roughly half of the amount necessary in order to achieve herd immunity and bring the virus under control, though it won’t eliminate it.
Q. In what order will the population be vaccinated?
A. The first phase will vaccinate 1.3 million healthcare and allied workers (receptionists at medical practises, cleaners, and so on.) In quarter one 2021 (January to March), those who are in regular contact with patients precede those who aren’t. In phase two, the elderly, key personnel (including police and possibly teachers) and patients with comorbidities (roughly 21 million people in total) will be vaccinated through quarters two and three (April 2021 to September 2021). The final phase (which will probably extend into 2022) targets the broader public, and will last until roughly half the population (40 million) has been vaccinated. We will probably vaccinate only people over the age of 18.
Q. What system is in place for the vaccine rollout? How will people register for their shot?
A. Planning is in place to identify healthcare workers in the private and public sectors. The process is being headed up by the department of health, and Discovery has offered to assist with gathering the details of frontline healthcare workers for the first phase of vaccination. Communication is expected to go out in the next few days asking doctors, nurses, pharmacists, and other frontline healthcare workers to submit their names and those of all workers in their practises to a database. This information will be fed into the vaccination planning system. A vaccine management and registration system for the broader public is also being developed along the same lines, prioritising patients based on their risk category and informing them when they can make a booking.
Q. Does it make a difference if I mix my vaccines and get shots from different suppliers?
A. Mixing them isn’t recommended. If your first dose is Pfizer, for example, the second shot should be as well. Mixing hasn’t been validated in any trial, and isn’t registered as a treatment.
Q. Are there any people who shouldn’t have the vaccine?
A. There are very few, rare contraindications that mean a person should avoid the vaccine. This includes people who are allergic to the constituent parts which make up the vaccine. Pregnant and nursing women are being advised to wait (depending on how pressing the need may be), but data hasn’t shown any impact of the vaccine on fertility. The vaccine is currently approved only for adults over 18, meaning children aren’t yet included in the vaccination programme.
Q. Vaccines have been tested, approved, and produced extremely rapidly. Does this mean that shortcuts have been taken where safety is concerned?
A. Coronaviruses aren’t new, and the technology that is being used in combatting them today has been developed over decades. The years of research mean that new products can be brought to the market faster than in the past. Moreover, all clinical data is reviewed by independent drug regulatory agencies for safety, and the panels of experts have no vested interest in the product directly. In fact, major pharmaceutical companies GlaxoSmithKline and Sanofi have delayed their vaccine to the end of the year because their results weren’t good enough and warranted some improvement.
When asked whether the panellists themselves would take the vaccine, each said they wanted to be first in line. A poll taken during the webinar indicated that 95% of viewers wanted the vaccine, 4% were unsure, and only 1% said they would decline it.
It’s believed that the first vaccines have already arrived in South Africa, and are undergoing quality testing before distribution.